Book Appointment Now

Stem Cell Therapy in Colombia: A Complete Medical Tourism Guide
Colombia has become a hotspot for stem cell therapy among medical tourists, but the reality is more complicated than the marketing suggests. Most treatments offered commercially remain unproven by rigorous clinical trials, despite what clinic websites might claim.
Who might consider this: Patients with knee osteoarthritis facing joint replacement, people with autoimmune conditions who’ve exhausted conventional options, anyone exploring regenerative medicine where standard treatments have failed.
Who should think twice: Patients considering high-risk spinal injections for neurological conditions, those expecting guaranteed results, people with conditions backed by minimal scientific evidence, anyone who can’t return for follow-up if complications arise.
The bottom line: While Colombia offers significant cost savings (50-70% less than US prices) and quality medical infrastructure, most stem cell therapies operate in a regulatory grey area. The International Society for Stem Cell Research considers most commercial offerings investigational at best.
What is stem cell therapy?
Mesenchymal stem cells (MSCs) are adult stem cells found in bone marrow, adipose tissue, and umbilical cord material. They can develop into bone, cartilage, and fat cells while potentially reducing inflammation and modulating the immune system through growth factors and other signaling molecules. Colombian clinics favor allogeneic cells from umbilical cord stem cells because there’s no harvesting surgery needed.
These cells theoretically support tissue repair and tissue regeneration through multiple mechanisms, but the gap between laboratory potential and real-world results is enormous. Clinics talk about “proprietary stem cell technology” and “enhanced potency protocols,” but these claims rarely appear in peer-reviewed research. It’s marketing speak for processes you can’t independently verify.
Side effects range from manageable to serious: joint pain and swelling (common), infection, allergic reactions to donor cells, blood clots with IV delivery. Long-term risks include potential tumor formation and severe immune reactions – effects we simply don’t understand yet.
Don’t get treatment if you have active cancer, severe immune problems, are pregnant or breastfeeding, have active infections, or serious bleeding disorders.
Why choose Colombia for stem cell therapy?
Cost drives most decisions. Colombian packages run $4,000-25,000 compared to $25,000-50,000+ for similar procedures in the US. Flight time from major US cities is 5-8 hours, visa-free entry lasts 90 days, and major cities have state-of-the-art facilities with English-speaking medical staff.
Colombia’s medical tourism reputation comes from JCI-accredited hospitals like Fundación Valle del Lili (Cali), Fundación Santa Fe de Bogotá, and Hospital Internacional de Colombia (Bucaramanga). Many stem cell clinics operate within or partner with these established medical facilities, though JCI accreditation doesn’t extend to specific regenerative medicine programs.
The regulatory gap: Colombia’s INVIMA (drug surveillance agency) has openly admitted that advanced cellular therapies don’t fit existing categories for medicines, devices, or anatomical components. Current INVIMA regulations cover tissue banking (Decree 2493/2004, Resolution 5108/2005) but not therapeutic applications. This regulatory framework allows clinics to offer medical “procedures” using processed cells regulated only at the tissue banking stage.
A 2024 proposed law seeks comprehensive regulatory frameworks for “terapias avanzadas,” confirming current gaps in oversight. Clinical trials provide the clearest regulatory pathway with INVIMA oversight, following international Good Clinical Practice standards.
Treatment delivery methods commonly offered:
- Intra-articular stem cell injections for joint conditions
- IV stem cell therapies for systemic conditions
- Intrathecal injections for neurological disorders (highest risk)
- Direct tissue injections for localized conditions
- Combination protocols with IV therapies and hyperbaric chamber sessions
Compare your options:
| Factor | Colombia | Mexico | Costa Rica | United States |
|---|---|---|---|---|
| Cost Range | $4,000-$25,000 | $3,000-$20,000 | $6,000-$30,000 | $25,000-$50,000+ |
| Regulation | Developing framework | Similar gaps | Limited oversight | FDA trials only |
| Infrastructure | Multiple JCI hospitals | Extensive JCI presence | Limited JCI facilities | Established standards |
| Language | Good English support | Variable support | Strong English support | None needed |
| Flight Time | 5-8 hours | 2-6 hours | 3-6 hours | Domestic |
Which conditions actually benefit from stem cell therapy?
The evidence varies wildly depending on what you’re treating. Colombian medical facilities commonly market treatments for dozens of conditions, but the scientific support differs dramatically:
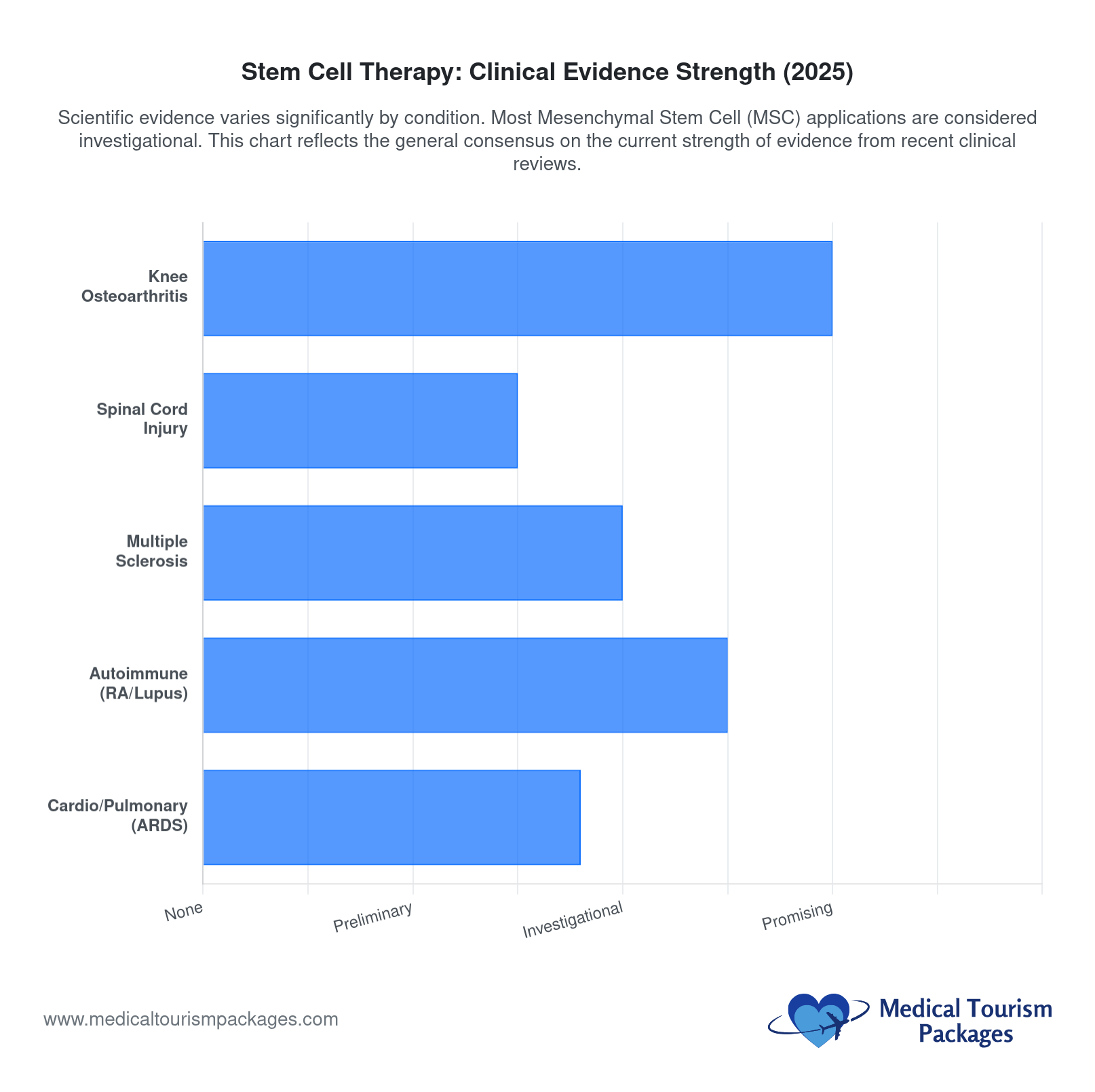
Orthopedic Applications – Best Evidence Base
Knee Osteoarthritis & Joint Pain – Moderate evidence
Treatment: Single intra-articular stem cell injection of 20-100 million MSCs for knee pain, hip pain, and other joint issues
Timeline: Gradual pain relief over 3-6 months, peak benefits possibly at 6-12 months
Data: 2024 review of ten randomized trials with 818 patients found MSCs beat hyaluronic acid for pain reduction and function improvement
Sports Injuries & Orthopedic Injuries – Limited evidence
Treatment: Direct stem cell injections for ligament, tendon, and muscle injuries
Applications: Back pain, neck pain, plantar fasciitis, wrist pain, degenerative disc disease
Evidence level: Mostly case studies and small trials, minimal randomized data
Neurological Disorders – High Risk, Minimal Evidence
Progressive Multiple Sclerosis – Very low evidence
Treatment: Intrathecal (spinal) injection of MSC-derived neural cells
Risks: Infection, bleeding, headache, potential spinal cord injury, extremely limited safety data
Data: One pilot study of six patients over 7.4 years – insufficient for treatment recommendations
Parkinson’s Disease & Other Neurological Conditions – Investigational only
Treatment: Various delivery methods including IV and intrathecal
Applications: Traumatic brain injury, spinal cord injuries, Cerebral Palsy
Evidence level: Early-stage research, no established efficacy
Autoimmune Disorders – Mostly Marketing
Systemic Autoimmune Diseases – Low evidence
Treatment: IV infusion of donor MSCs targeting immune system dysfunction
Applications: Lupus, rheumatoid arthritis, Crohn’s disease, multiple sclerosis
Data: 2025 review of 244 stem cell trials found 83.6% in early Phase I-II stages
Cardiovascular Applications – Safety Concerns
Heart Disease & Cardiovascular Diseases – Low evidence, concerning safety
Treatment: Various delivery methods for ischemic heart conditions
Safety data: 80 deaths among 1,408 MSC recipients across 33 trials, 33% showed no benefit
Aesthetic & Anti-Aging Applications
Cosmetic Treatments – Minimal evidence
Applications: Hair restoration, facial rejuvenation, wrinkle reduction, scar reduction
Treatment: Local stem cell injections or IV therapies
Evidence level: Mostly testimonials and before/after photos
Sexual Health – Very limited data
Applications: Erectile dysfunction, enhanced sexual performance, vaginal rejuvenation
Evidence level: Small studies, unclear long-term effects
Specialized Applications
COVID-19 ARDS – Moderate evidence (hospital settings only)
Application: IV MSCs for hospitalized COVID-19 patients with severe lung inflammation
Context: Emergency use in acute care settings, not preventive treatment
Chronic Pain Conditions – Variable evidence
Applications: Fibromyalgia, chronic fatigue, general pain relief
Treatment protocols: Often combined with IV therapies and hyperbaric chamber sessions
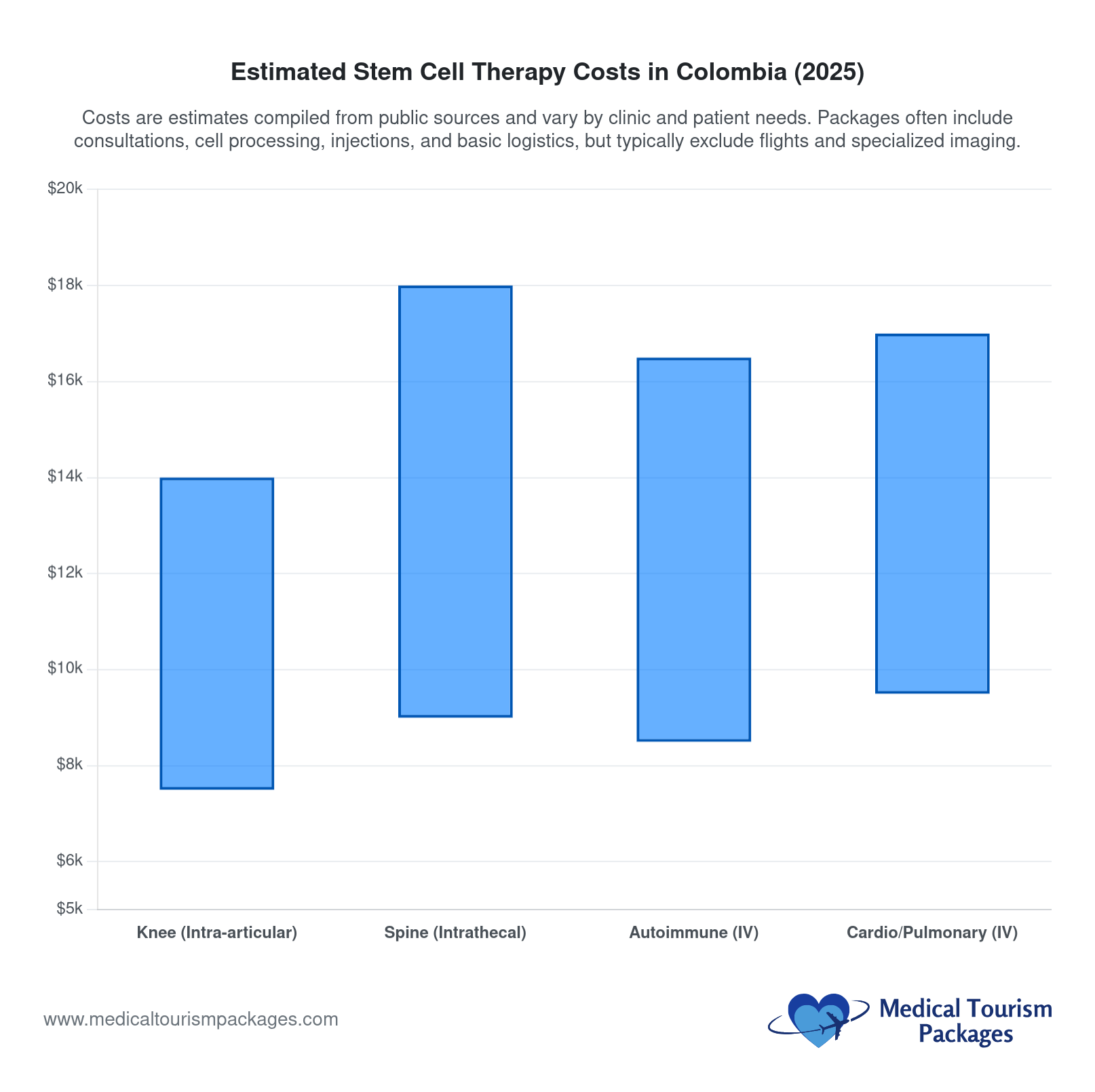
How much does stem cell therapy cost in Colombia?
| Treatment Type | Price Range (USD) | Typical Inclusions | Major Exclusions |
|---|---|---|---|
| Single Joint | $5,500-$12,000 | Cell processing, injection, consultations, hotel (3-5 nights), VIP transfer | Flights, MRI, comprehensive laboratory tests, complications |
| Systemic IV | $10,000-$18,000 | Cell processing, IV infusion, wellness IVs, hotel (5-7 nights) | Flights, CT scans, extensive pre-treatment evaluations, follow-up |
| Neurological | $15,000-$25,000+ | Multiple infusions, hyperbaric chamber, extended stay | Flights, specialized imaging, emergency care |
| Anti-Aging/Aesthetic | $8,000-$15,000 | Cell-based treatments, IV therapies, consultation | Flights, multiple sessions, post-treatment care |
Popular clinic networks offering comprehensive packages include facilities in Bogotá, Medellín, and Cali, with some clinics like international stem cell therapy clinics providing personalized treatment plans based on individual patient needs and specific conditions.
Each personalized treatment plan typically includes comprehensive pre-treatment evaluations, customized cell preparation protocols, and coordinated post-treatment care. Many state-of-the-art facilities now offer combination approaches integrating donor stem cell therapy with complementary treatments like hyperbaric chamber sessions and specialized IV therapies.
Budget for extras: international flights ($500-2,000+), comprehensive laboratory tests and CT scans, travel insurance with medical evacuation ($200-800), companion expenses, post-treatment rehabilitation, and complication management.
Payment terms typically require 10-30% deposits with balance due on arrival. Credit cards provide dispute protection. Many facilities offer VIP transfer services and concierge-style coordination for international patients.
How do you choose a safe stem cell clinic?
Quality facilities should provide cell viability percentages (above 85%), exact cell counts (20-300 million depending on application), and sterility testing documentation. GMP laboratory certification is necessary – verify it independently.
20-Point Vetting Checklist
Regulatory Compliance (Non-negotiable):
- INVIMA registration number and current status
- Clinical trial registration (NCT number) if applicable
- Independent ethics committee approval documentation
- GMP certification for cell processing laboratory
- Professional liability insurance verification
Clinical Evidence & Safety: 6. Published studies on their specific cell product 7. Documented success and complication rates for your condition 8. Clear risk profiles with detailed informed consent 9. Patient follow-up protocols and compliance rates 10. Systematic adverse event reporting and management
Treatment Quality: 11. Exact cell count and viability percentage specifications 12. Donor screening and safety testing protocols 13. Sterility and quality control testing documentation 14. Physician credentials and experience in cellular therapies 15. Emergency protocols for adverse reactions
Business Practices: 16. Fully itemized quotes with all potential costs 17. Clear policies for treatment failures or complications 18. Coordination protocols with home physicians 19. Medical records privacy and patient data protection 20. Post-treatment emergency support and accessibility
Red flags: Unsubstantiated cure claims, inability to provide regulatory documentation, excessive condition lists without supporting evidence, pressure for immediate decisions, unwillingness to communicate with home physicians.
Where should you get treated and what’s the process like?
Bogotá: Capital with comprehensive healthcare infrastructure, El Dorado Airport, 2,640m elevation (may cause altitude adjustment), extensive English-speaking medical support.
Medellín: “City of Eternal Spring,” José María Córdova Airport with 5-7 hour direct US flights, 1,500m elevation, temperate climate, developed medical tourism infrastructure.
Cali: Home to JCI-accredited Fundación Valle del Lili, smaller commercial stem cell market, Alfonso Bonilla Aragón Airport (fewer direct international flights).
Timeline
Pre-Travel (4-8 weeks): Virtual consultation, medical records submission, home physician clearance, required lab tests, travel insurance, flexible flight booking.
Days 1-2: Airport pickup, hotel orientation, comprehensive evaluation, final imaging/labs, informed consent, treatment confirmation.
Days 3-4: Cell preparation (24-48 hours), treatment day (2-4 hour monitoring), immediate recovery with activity restrictions.
Day 5+: Daily check-ins week 1, weekly consultations month 1, formal assessments at 2-3 months and 6+ months.
Set realistic expectations: Benefits, if they happen at all, typically begin 4-12 weeks post-treatment with peak effects at 3-6 months. Not all patients respond; some experience no change or complications.
What do you need to know about traveling to Colombia for treatment?
Entry requirements: US, Canadian, UK citizens enter visa-free for 90 days (extendable to 180 total). Need valid passport, return travel proof, sufficient funds demonstration.
Safety precautions: Government recommends “high degree of caution” due to crime. Maintain low profile, avoid displaying valuables, use ride-sharing over street taxis, never leave drinks unattended (scopolamine risk).
Accommodation needs: Proximity to clinic/emergency facilities, 24-hour security, English-speaking staff, reliable internet, quiet environment, room service, wheelchair accessibility if needed.
Financial: Credit cards widely accepted (notify bank), US dollars accepted but pesos offer better rates, many clinics prefer wire transfers for large payments.
What are the risks and how do you handle emergencies?
Immediate risks by delivery method:
- Joint injections: Pain/swelling (common), infection, cartilage damage (rare)
- IV infusion: Flu-like symptoms, blood clot risk, severe allergic reactions
- Spinal delivery: Highest risk – headache, back pain, infection, neurological injury, death
Long-term safety concerns: Uncontrolled cell growth/tumor formation, autoimmune reactions, unknown fertility effects.
Insurance reality: Most US health insurance excludes experimental treatments abroad and their complications. Medical evacuation coverage ($50,000-100,000+) is necessary.
Emergency protocols:
- Contact Colombian clinic: New symptoms at injection sites, recovery questions
- Seek local emergency care: High fever, neurological symptoms, chest pain, breathing difficulties
- 24/7 support: Establish clear escalation procedures and maintain comprehensive treatment records
Post-treatment care: Coordinate with home healthcare providers familiar with regenerative medicine. Plan structured rehabilitation, document outcomes objectively, maintain long-term monitoring for delayed complications.
Frequently Asked Questions
How effective are stem cell treatments for my condition?
Effectiveness varies by condition. Knee osteoarthritis has moderate support from clinical trials, but conditions like Alzheimer’s, ALS, and autism have minimal evidence. Most commercial stem cell therapies remain investigational, with benefits unproven in rigorous trials.
How long before I see results?
If benefits occur, they usually begin 4–12 weeks after treatment, often peaking around 3–6 months. Some patients note improvement up to 12 months; others see no change or a temporary worsening. Don’t expect immediate results.
What’s the success rate?
Most clinics don’t publish comprehensive, standardized outcome data. Reported success varies widely by condition, patient factors, and how “success” is defined. Many patients experience partial improvement rather than dramatic change.
Are multiple treatments necessary?
It depends on your condition and response. Some patients do well with a single treatment; others may need additional sessions. Be cautious of clinics that push costly series upfront without allowing time to assess initial results.
Is stem cell therapy safe?
Generally low risk, but not risk-free. Immediate risks include infection and allergic reactions; long-term risks are less understood (e.g., tumor formation, immune issues). Risk varies by delivery method—spinal injections carry higher risk than joint injections.
What are the most common side effects?
Flu-like symptoms (fatigue, low-grade fever), localized pain and swelling at injection sites, and temporary worsening of baseline symptoms are common and usually resolve within 48–72 hours. Serious complications—while less common—can include severe allergic reactions, infections, and systemic issues.
Can stem cell therapy make my condition worse?
Yes—though uncommon, treatments can worsen an underlying condition, trigger autoimmune reactions, or mask disease progression. Careful candidate selection and ongoing monitoring are essential.
Are treatments approved by Colombian health authorities?
Most commercial stem cell treatments operate in a regulatory grey area. Tissue banking is regulated, but therapeutic use of processed cells generally falls outside standard drug approval pathways in Colombia.
Will my insurance cover complications from treatment in Colombia?
Most U.S. health plans don’t cover experimental treatments abroad or their complications. Verify coverage with your insurer and consider supplemental medical tourism insurance. Medical evacuation coverage is particularly important.
How do I choose a reputable clinic?
Use a robust vetting checklist. Prioritize clinics with regulatory documentation, published research, clear informed consent, and transparent outcome tracking. Avoid facilities that make sweeping cure claims or pressure quick decisions.
What type of stem cells are used in Colombian clinics?
Most clinics use donor mesenchymal stem cells (MSCs) derived from umbilical cord Wharton’s jelly. Donor cells avoid patient harvesting but may carry higher immune reaction risks than autologous (your own) cells.
Why are prices so variable between clinics?
Costs reflect cell processing complexity, facility overhead, included services, marketing spend, and protocol differences. Wide price ranges also mirror quality variation and what’s bundled in each package.
How long should I stay in Colombia?
Plan 3–7 days for simpler treatments and 10–14 days for complex protocols. Build in extra time for possible complications or added consultations. Arriving 1–2 days early helps with acclimatization.
What if I need emergency care after returning home?
Set up care with your home providers before travel. Carry full treatment records and clinic contacts, and ensure your medical team knows about the experimental therapy and potential complications to monitor.
What key terms should you understand?
Adult Stem Cells: Stem cells found in mature tissues like bone marrow and adipose tissue, as opposed to embryonic stem cells derived from early-stage embryos. Most commercial treatments use adult stem cells for safety and ethical reasons.
Adipose Tissue: Fat tissue containing mesenchymal stem cells that can be harvested for autologous therapies. Some clinics extract cells from patient’s fat tissue rather than using donor cells.
Allogeneic: Stem cells from a donor rather than the patient’s own body. Most Colombian clinics use allogeneic cells from umbilical cord tissue.
Autologous Therapies: Treatments using the patient’s own stem cells, typically harvested from bone marrow or adipose tissue through minor surgical procedures.
Bone Marrow: Traditional source of mesenchymal stem cells, requiring a more invasive harvesting procedure compared to adipose tissue or umbilical cord sources.
Cell-Based Treatments: Broader term encompassing various stem cell therapies and other cellular interventions for tissue repair and regeneration.
cGMP: Current Good Manufacturing Practices – quality standards for pharmaceutical production, including cell processing facilities.
Embryonic Stem Cells: Pluripotent cells derived from early-stage embryos, rarely used in commercial treatments due to ethical and safety concerns. Most Colombian clinics use adult stem cells instead.
Growth Factors: Proteins released by stem cells that promote tissue repair, reduce inflammation, and stimulate regeneration in damaged tissues.
INVIMA: Colombia’s National Institute for Drug and Food Surveillance, the primary regulatory authority overseeing medical products and INVIMA regulations governing tissue banking.
Intrathecal: Delivery method involving injection directly into cerebrospinal fluid via spinal tap. Highest-risk administration route for stem cells.
ISSCR: International Society for Stem Cell Research, the leading global organization providing scientific and ethical guidelines for stem cell research and clinical applications.
JCI: Joint Commission International – international healthcare accreditation organization indicating high-quality hospital systems but doesn’t specifically validate stem cell programs.
Mesenchymal Stem Cells (MSCs): Adult stem cells found in various tissues with ability to differentiate into bone, cartilage, and fat cells while providing anti-inflammatory effects through immune system modulation.
Stem Cell Research: Scientific investigation into the therapeutic potential of various stem cell types, conducted by research institutions and clinical facilities worldwide.
Transplanting Stem Cells: The process of introducing stem cells into patients through various delivery methods including injection, infusion, or surgical implantation.
Umbilical Cord Tissue: Source tissue containing Wharton’s jelly, from which many Colombian clinics derive their donor stem cells for treatments.
Wharton’s Jelly: Gelatinous substance in umbilical cords containing mesenchymal stem cells. Primary cell source for most Colombian stem cell clinics offering allogeneic treatments.
How do we vet these clinics?
Medical Tourism Packages uses a multi-phase evaluation process prioritizing patient safety and evidence-based practice over marketing claims.
We verify INVIMA registration, clinical trial authorizations, and ethics committee oversight. Our medical advisory team evaluates published research records, outcome data, and peer-reviewed contributions. We conduct independent verification of laboratory certifications (GMP, ISO), facility accreditations, and quality control protocols.
Data sources: INVIMA databases, ClinicalTrials.gov, PubMed searches, direct certification verification, Colombian medical board records.
Disqualifying factors: Unsubstantiated cure claims, inability to provide regulatory documentation, serious safety violations, misleading marketing, unwillingness to participate in vetting.
We re-evaluate clinics annually and following significant changes. Medical Tourism Packages doesn’t accept payments from clinics for favorable evaluations. Revenue comes from patient consultation fees and transparent service charges, not provider marketing arrangements.
How do you get started?
Schedule a consultation with our medical advisory team to review your medical history, current condition, treatment alternatives, and candidacy for stem cell therapy. We provide vetted clinic options meeting our safety standards, facilitate direct communication with selected clinics, and offer comprehensive treatment planning including travel logistics and emergency protocols.
Contact options: Free initial consultation, detailed medical review with personalized clinic recommendations, treatment coordination with full-service support, 24/7 emergency assistance.