Book Appointment Now
Stem Cell Transplants in Colombia: Types, Costs, Leading Clinics, and Treatment Process
Colombia has emerged as a leading destination for stem cell transplant procedures, combining world-class medical expertise with significantly lower costs than North American and European alternatives. The country’s healthcare system offers both autologous transplants, which use the patient’s own stem cells, and allogeneic transplants, which rely on donor cells from family members or matched donors.
Patients seeking stem cell treatment in Colombia can expect comprehensive care at internationally accredited facilities, with procedures costing 60-70% less than similar treatments in the United States. The nation’s medical infrastructure supports complex hematologic conditions including leukemia, lymphoma, multiple myeloma, and various blood disorders, while also advancing research in regenerative medicine applications.
This guide examines the transplant options available, associated costs, top-rated medical centers, and what patients can expect throughout their treatment journey in Colombia’s rapidly evolving stem cell therapy landscape.
What are stem cell transplants in Colombia?
Colombia’s stem cell treatment landscape operates through two distinct markets with different regulatory standards and applications. The first involves highly regulated hematopoietic stem cell transplantation (HSCT), which treats serious blood and immune system disorders. The second encompasses commercial regenerative medicine using mesenchymal stem cells (MSCs) for a broader range of conditions.
HSCT procedures include traditional bone marrow transplants that address immune deficiencies, aplastic anemia, and malignant diseases like leukemia and lymphoma. These treatments follow strict international protocols and require extensive patient evaluation, donor matching, and specialized facilities with isolation units.

Commercial stem cell therapies primarily utilize MSCs derived from sources like adipose tissue or umbilical cord blood. These treatments target orthopedic injuries, autoimmune disorders, neurological diseases, and anti-aging applications. While less regulated than HSCT, reputable clinics maintain quality standards for cell processing and patient safety.
Stem Cells Colombia, one of the country’s prominent regenerative medicine centers, serves approximately 200 patients annually. The clinic represents the growing commercial sector that attracts international patients seeking alternative treatments not widely available in their home countries.
What types of stem cell transplants are available in Colombia?
Colombia offers three primary types of stem cell transplants, each with distinct advantages and clinical applications.
Autologous transplants use cells harvested directly from the patient’s own adipose tissue or bone marrow. This approach carries a lower risk of immune rejection since the cells originate from the patient’s body. However, it requires an initial harvesting procedure, which adds time and complexity to the treatment process.
Allogeneic transplants source stem cells from external donors, most commonly from umbilical cord tissue. Colombia provides access to culture-expanded allogeneic cells, which remain restricted in the United States and Europe. These donor-derived cells are preferred for their youthful characteristics and high potency, while eliminating the need for patient harvesting procedures.
Haploidentical transplants represent a specialized form of allogeneic transplantation using partially matched family donors. This option proves particularly valuable for pediatric patients, with Colombian medical centers reporting a 72% survival rate at one year for haploidentical donor transplants in children.
The availability of culture-expanded allogeneic cells distinguishes Colombia’s stem cell market from more restrictive regulatory environments, providing patients with treatment options that may not be accessible in their home countries.
Which conditions can be treated with stem cell transplants in Colombia?
Colombian medical centers treat a wide range of conditions through stem cell transplantation, with varying levels of clinical evidence and regulatory approval.
Blood cancers represent the most established application for stem cell therapy in Colombia. Multiple myeloma and other malignant hematologic diseases receive treatment through HSCT procedures at major medical centers. Fundación Valle de Lili has performed 1,437 bone marrow transplant procedures since 1998, demonstrating the country’s extensive experience with these established treatments.
Autoimmune diseases constitute a growing area of stem cell research and treatment. A 2025 review of 244 stem cell trials for autoimmune conditions found that 83.6% remain in Phase I-II safety testing stages. Medical authorities classify these treatments as “low evidence” or “investigational only,” indicating that while promising, these applications lack definitive proof of efficacy.
Genetic disorders, particularly inborn errors of immunity, show measurable treatment outcomes in Colombian facilities. Pediatric patients with identical donors achieve an 80% one-year survival rate, while those receiving cord blood sources show a 63% survival rate. The overall five-year survival rate for pediatric patients with genetic immune disorders reaches 63%.
These success rates reflect Colombia’s advancing capabilities in treating complex genetic conditions through specialized transplant protocols.
What is the difference between stem cell transplants and stem cell therapy?
The terms “stem cell transplant” and “stem cell therapy” describe distinct medical approaches with different objectives, complexity levels, and regulatory oversight.
Stem cell transplants involve replacing a patient’s diseased or damaged bone marrow with healthy stem cells from either the patient themselves or a donor. These procedures treat serious blood cancers, immune deficiencies, and genetic disorders through intensive medical interventions. Transplants require hospitalization, isolation units, and extensive pre-conditioning treatments that suppress the patient’s immune system.
Stem cell therapy encompasses a broader range of treatments using stem cells to repair, replace, or regenerate damaged tissues and organs. These therapies typically involve injections or infusions of processed stem cells into specific areas or systemically throughout the body. Most stem cell therapies are outpatient procedures with minimal recovery time.
Clinical applications differ significantly between the two approaches. Transplants focus on life-threatening hematologic conditions with established treatment protocols and decades of clinical evidence. Therapies target a wider range of conditions including orthopedic injuries, autoimmune disorders, and degenerative diseases, though many applications remain experimental.
Regulatory status also varies considerably. Stem cell transplants operate under strict medical guidelines with standardized protocols worldwide. Many stem cell therapies exist in regulatory gray areas, particularly commercial applications that may lack comprehensive clinical validation.
Understanding this distinction helps patients make informed decisions about appropriate treatment options for their specific conditions.
What are the costs of stem cell transplants in Colombia?
Stem cell treatment costs in Colombia vary significantly based on the procedure complexity and treatment duration. International patients should budget for both medical expenses and travel-related costs.
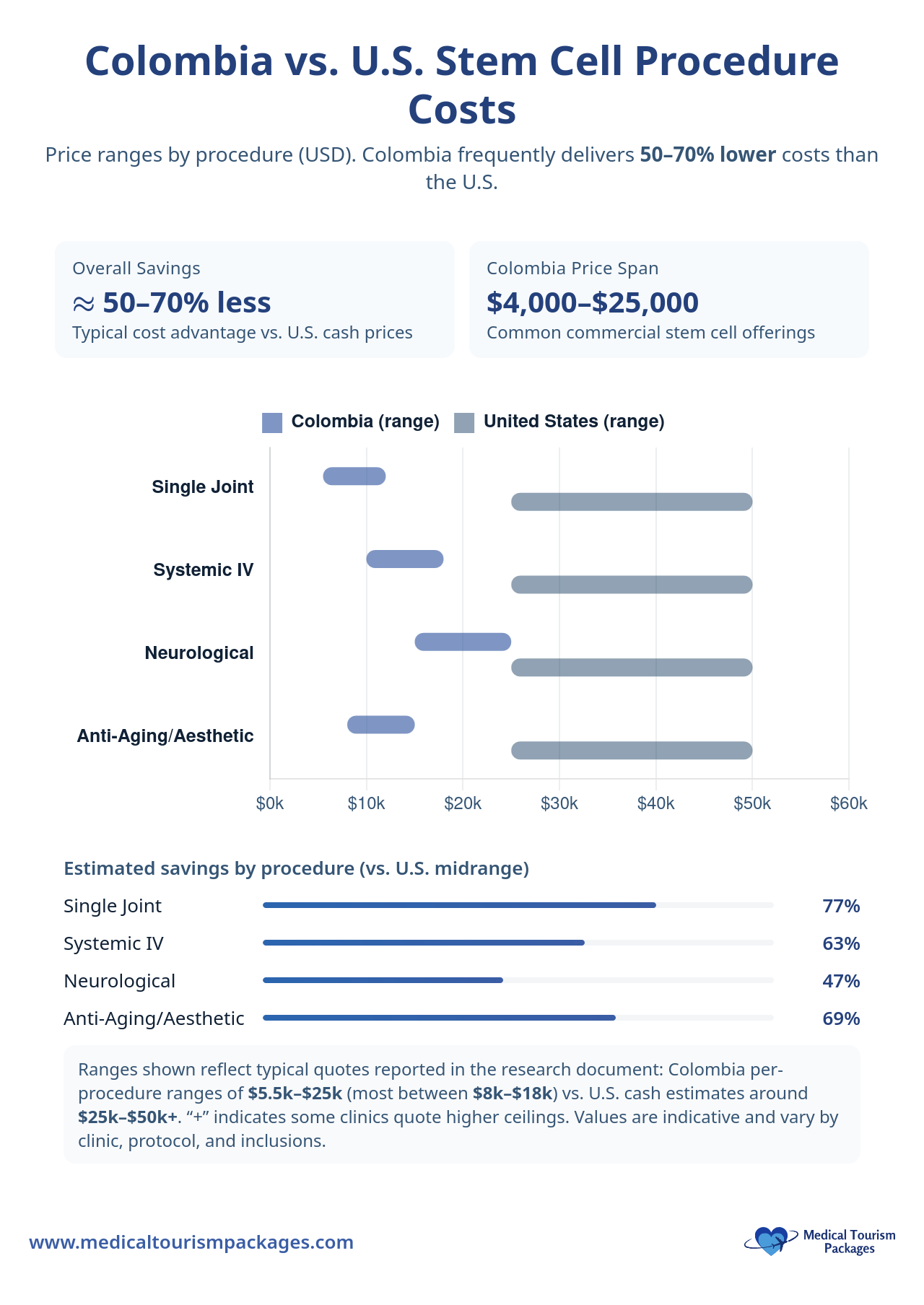
| Treatment Type | Price Range (USD) | Typical Inclusions | Major Exclusions |
| Single Joint Injection | $5,500-$12,000 | Cell processing, injection, consultations, hotel (3-5 nights) | Flights, MRI, comprehensive lab tests |
| Systemic IV | $10,000-$18,000 | Cell processing, IV infusion, wellness IVs, hotel (5-7 nights) | Flights, CT scans, extensive pre-treatment evaluations |
| Neurological | $15,000-$25,000+ | Multiple infusions, hyperbaric chamber, extended stay | Flights, specialized imaging, emergency care |
| Anti-Aging/Aesthetic | $8,000-$15,000 | Cell-based treatments, IV therapies, consultation | Flights, multiple sessions, post-treatment care |
Additional costs require separate budgeting considerations. International flights typically range from $500-2,000+ depending on origin location. Travel insurance with medical evacuation coverage is strongly recommended for international patients. Most clinics require a 10-30% deposit upfront to secure treatment dates.
Standard health insurance policies do not cover these treatments, making patients responsible for all costs. The total investment often represents 60-70% savings compared to similar procedures in North American facilities, when such treatments are available.
How do you get a stem cell transplant in Colombia?
Getting a stem cell transplant in Colombia requires careful research, proper vetting of facilities, and coordinated planning between your home country and Colombian medical teams.
Finding qualified centers demands thorough evaluation of each facility’s credentials and capabilities. Use this vetting checklist to assess potential treatment centers:
- INVIMA registration number and current status
- cGMP certification for cell processing laboratory
- Independent ethics committee approval documentation
- Clinical trial registration (NCT number) if applicable
- Documentation of cell viability percentages (above 85%)
- Exact cell counts documentation
- Donor screening and sterility testing protocols
Initial consultation process begins 4-8 weeks before travel. Patients participate in virtual consultations and submit complete medical records for review. Some clinics require medical clearance from the patient’s home physician before approving treatment.
Treatment timeline involves an on-site stay of 5-14 days depending on the procedure complexity. Autologous cell processing requires 24-48 hours in specialized laboratories to prepare the patient’s harvested cells. Most facilities offer all-inclusive packages that include VIP airport transfers and concierge assistance throughout the stay.
Recovery period varies by treatment type. Orthopedic treatments typically show gradual improvement over 3-6 months, with peak benefits occurring at 6-12 months post-treatment. Clinics schedule remote check-ins at 1, 3, 6, and 12 months. Emergency protocols require patients to contact their Colombian clinic immediately for any new symptoms or complications.
What are the leading stem cell transplant centers in Colombia?
Colombia’s major cities host internationally recognized stem cell treatment centers, each offering specialized expertise and varying approaches to regenerative medicine.
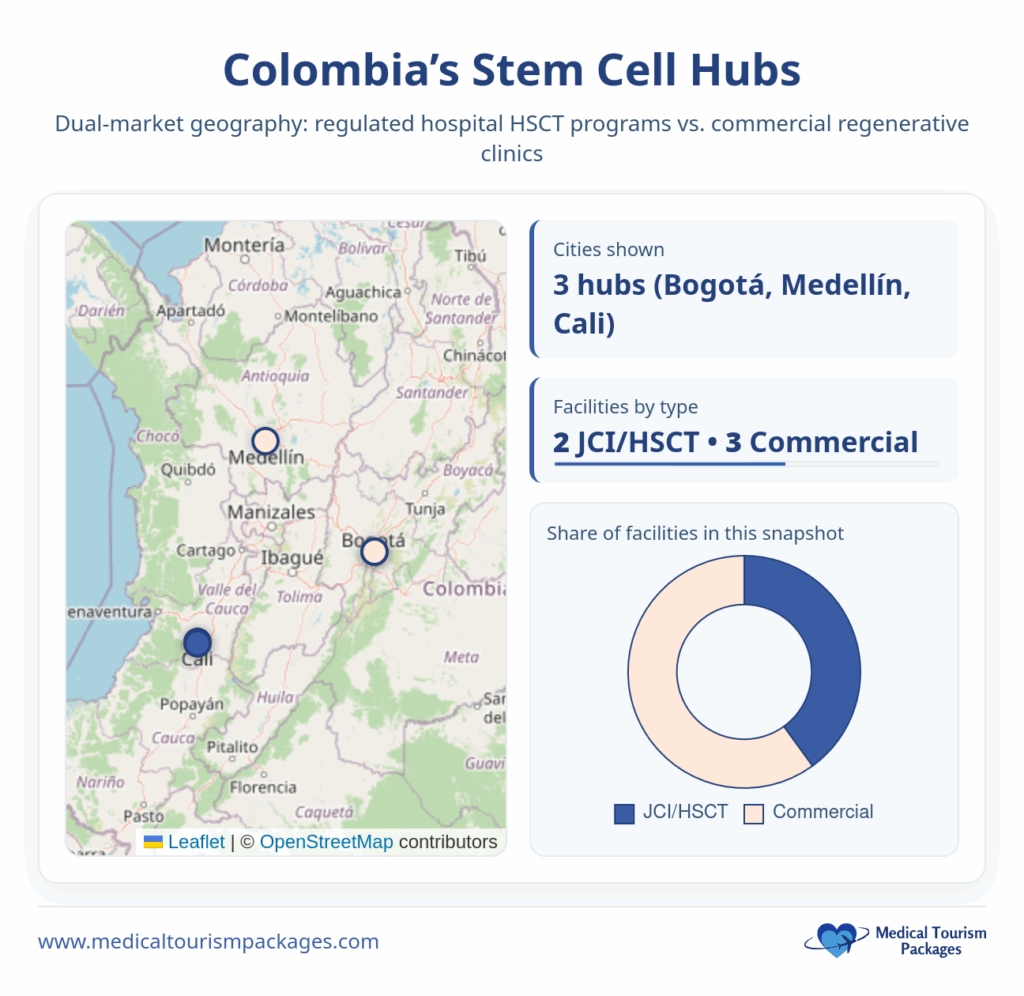
Cali:
- Fundación Valle del Lili: JCI-accredited facility with extensive experience in stem cell transplantation. The center has performed 1,437 bone marrow transplants since 1998 through its specialization program with Icesi University. Research director Dr. Diego Medina Valencia leads their transplant program.
- Stem Cells Kyron: Led by Dr. Carlos Eduardo Rojas Martinez, this facility specializes in umbilical cord tissue MSCs and provides comprehensive lab documentation for all treatments.
Bogotá:
Instituto Nacional de Cancerología: Features Dr. Leonardo Gonzalez, founder of the International Stem Cell Institute and certified specialist in Regenerative and Anti-Aging Medicine. The institute pioneered stem cell treatments for Crohn’s disease and heart conditions in Colombia.
Medellín:
- BioXcellerator: Markets itself as a “5-star experience” with integrated clinic, hotel, and shopping facilities under one roof. The center focuses on high-potency MSCs and exosome therapies.
- Hospital Pablo Tobón Uribe: Established medical center offering traditional stem cell transplant services.
- Clínica Las Américas: Regional facility providing stem cell treatments with focus on local patient populations.
Each center maintains different specializations, with some focusing on traditional HSCT procedures while others emphasize commercial regenerative medicine applications.
What are the success rates for stem cell transplants in Colombia?
Success rates for stem cell transplants in Colombia vary significantly based on treatment type, with established procedures showing higher efficacy than experimental applications.
Success Rates by Treatment Type
| Treatment Type | Success Rate | Evidence Level | Notes |
| Orthopedic (knee osteoarthritis) | 70-75% | Moderate evidence (10 randomized trials) | MSCs more effective than hyaluronic acid |
| Pediatric HSCT (identical donors) | 80% 1-year survival | High evidence | 5-year survival 63% |
| Pediatric HSCT (haploidentical) | 72% 1-year survival | High evidence | Established protocols |
| Cardiovascular | 33% show no benefit | Low evidence | 80 deaths among 1,408 patients in trials |
| Autoimmune | 83.6% still in Phase I-II | Investigational | Limited long-term data |
Traditional HSCT procedures demonstrate the strongest success rates, particularly for pediatric patients with genetic immune disorders. These established protocols benefit from decades of refinement and international standardization.
Orthopedic applications show promising results for knee osteoarthritis, with MSC treatments outperforming conventional hyaluronic acid injections in controlled studies. The 70-75% success rate reflects moderate-quality evidence from multiple randomized trials.
Experimental treatments for cardiovascular and autoimmune conditions remain largely investigational. The high percentage of autoimmune trials still in early-phase testing indicates insufficient data for definitive success rate assessments. Cardiovascular applications show concerning safety signals, with notable mortality rates in clinical trials.
Patients should carefully evaluate evidence levels when considering treatment options, as success rates correlate strongly with the maturity of clinical research supporting each application.
What are the requirements for international patients seeking stem cell transplants in Colombia?
International patients must satisfy specific visa, financial, and documentation requirements before receiving stem cell treatment in Colombia.
Documentation Requirements:
Visa requirements: Depends on nationality and treatment duration. Citizens from over 90 countries, including the United States, Canada, European Union members, Australia, and most Latin American nations, can enter Colombia visa-free for up to 90 days for tourism purposes. The Special Temporary Visa for Medical Treatment is only required for extended treatments that cannot be completed within the standard 90-day tourist period, costing $50 plus processing fees and taking several weeks to approve.
Medical insurance: Stem cell treatments remain universally out-of-pocket expenses, with no coverage from standard health insurance policies. Travel insurance with medical evacuation coverage is strongly recommended for international patients, as complications may require emergency transport to home country facilities.
Language services: Most reputable clinics include translation services within their all-inclusive treatment packages. Patient coordinators provide personalized support throughout the treatment process, helping navigate medical appointments, accommodation arrangements, and local logistics.
The Special Temporary Visa for Medical Treatment requires advance planning due to processing delays. Patients should initiate visa applications immediately after treatment approval to avoid scheduling conflicts. Financial documentation must demonstrate sufficient resources to cover both medical expenses and extended stay costs, as treatments may require longer recovery periods than initially anticipated.
Colombian medical institutions must provide official certification supporting the visa application, establishing the medical necessity and treatment plan before approval.
How is stem cell transplant regulated in Colombia?
Colombia’s regulatory framework for stem cell transplants operates within a complex legal landscape that creates both opportunities and uncertainties for patients and providers.
INVIMA’s role in overseeing stem cell treatments faces significant challenges due to regulatory gaps. Advanced cellular therapies don’t fit existing legal categories designed for traditional medicines or medical devices, creating a “regulatory grey area” for commercial clinics. Most clinics currently operate under tissue banking regulations established by Decree 2493/2004 and Resolution 5108/2005, which were not specifically designed for cellular therapy applications.
Legal requirements remain in flux as Colombian lawmakers work to address regulatory uncertainties. A 2024 proposed law for “terapias avanzadas” (advanced therapies) remains pending approval, which would establish clearer guidelines for stem cell treatments. INVIMA has publicly acknowledged the current regulatory gap and the need for updated legislation to properly govern cellular therapy practices.
Culture-expanded cells, which remain restricted in the United States and Europe due to strict FDA and EMA regulations classifying them as drugs requiring clinical trials, are available in Colombia under the current regulatory framework. This regulatory difference allows Colombian clinics to offer treatments using laboratory-expanded MSCs that would require years of clinical trials in more restrictive jurisdictions.
Ethical oversight varies significantly among Colombian facilities, with many clinics lacking full documentation requirements standard in developed nations. While some centers maintain independent ethics committees and follow international guidelines, others operate with minimal oversight. Patients should verify that their chosen facility provides ethics committee approval documentation and follows informed consent protocols aligned with Declaration of Helsinki principles.
Stem cell tourism considerations require careful evaluation of risks associated with unproven treatments and limited follow-up care. Many international patients travel to Colombia for experimental therapies not approved in their home countries, potentially exposing themselves to treatments with unverified safety profiles. The lack of coordinated follow-up care between Colombian providers and home country physicians can complicate management of adverse events or treatment complications.
Comparison with regulatory standards reveals significant differences between Colombia and other jurisdictions. The United States FDA requires extensive clinical trials for cell-based therapies, with only a handful of approved treatments. European regulations under the European Medicines Agency similarly demand rigorous testing and manufacturing standards. Other Latin American countries like Mexico and Panama offer even less regulatory oversight than Colombia, while Brazil and Argentina maintain stricter controls comparable to European standards.
This regulatory uncertainty allows some clinics to operate with limited oversight while others voluntarily adopt international standards. Patients should verify that their chosen facility maintains proper tissue banking licenses and follows established safety protocols, even in the absence of specific cellular therapy regulations.
The pending advanced therapy legislation aims to align Colombia’s regulatory framework with international standards, potentially improving patient safety and treatment standardization once implemented. Until comprehensive regulations are enacted, patients must exercise due diligence in selecting reputable facilities that prioritize safety and ethical practices despite the permissive regulatory environment.
Get started with confident care
Ready to explore Colombia’s stem cell options with confidence? Our facilitation team handles the essentials, from matching you with accredited centers and bilingual care coordinators to organizing records reviews, transparent pricing, travel logistics, and post visit check ins.
Tell us about your diagnosis, timeline, and budget and we will build a safe, tailored plan. Begin your personalized medical tourism package today: Contact our team.
Frequently Asked Questions
What are stem cell transplants in Colombia?
Colombia has two parallel markets: (1) highly regulated hematopoietic stem cell transplantation (HSCT) for blood and immune disorders using strict international protocols, and (2) commercial regenerative medicine with mesenchymal stem cells (MSCs) for orthopedic, autoimmune, neurological, and anti-aging uses. HSCT requires donor matching, conditioning, and isolation units; commercial MSC clinics vary in oversight but reputable centers maintain quality and safety standards.
What types of stem cell transplants are available in Colombia?
Three main types: Autologous (your own cells from bone marrow or adipose; lower rejection risk but requires harvesting), Allogeneic (donor cells—often umbilical cord tissue; culture-expanded cells are available in Colombia but restricted in the U.S./EU), and Haploidentical (partially matched family donors), which is especially useful in pediatrics.
Which conditions can be treated with stem cells in Colombia?
Established (HSCT): leukemia, lymphoma, multiple myeloma, and other hematologic diseases at accredited centers (e.g., Fundación Valle del Lili has performed 1,437 bone marrow transplants since 1998). Investigational/Regenerative: autoimmune disorders (most trials still Phase I–II), orthopedic injuries (notably knee osteoarthritis), neurological conditions, and genetic immune disorders with documented pediatric outcomes.
How much do stem cell treatments cost in Colombia?
Typical ranges (USD): single joint injection $5,500–$12,000; systemic IV $10,000–$18,000; neurological $15,000–$25,000+; anti-aging/aesthetic $8,000–$15,000. Packages may include consultations, cell processing, and hotel nights; flights, advanced imaging, and extensive labs are usually extra. A 10–30% deposit is common. Total costs are often 60–70% less than U.S. equivalents.
How do international patients arrange stem cell treatment in Colombia?
Start 4–8 weeks before travel with virtual consults and complete medical record review (some clinics require local physician clearance). Plan a 5–14 day stay; autologous cell processing typically takes 24–48 hours. Expect remote follow-ups at 1, 3, 6, and 12 months. Vet clinics for INVIMA registration, cGMP labs, ethics approval, trial registration (if applicable), >85% viability, exact cell counts, and rigorous donor screening/sterility testing.
What are the success rates and evidence levels?
The success rates for stem cell transplant treatments are
- HSCT: pediatric identical donors ~80% 1-year survival (63% at 5 years); haploidentical ~72% 1-year; cord blood ~63% 1-year.
- Orthopedic (knee OA): ~70–75% success; MSCs outperform hyaluronic acid (moderate evidence).
- Autoimmune: ~83.6% of trials remain Phase I–II (investigational).
- Cardiovascular: mixed/low evidence; ~33% show no benefit with notable safety signals reported.
How are stem cell treatments regulated in Colombia?
HSCT follows strict medical standards. Commercial MSC therapies operate under tissue banking rules (Decree 2493/2004; Resolution 5108/2005) amid a regulatory grey area acknowledged by INVIMA; a 2024 advanced therapies bill is pending. Colombia permits culture-expanded MSCs that are restricted in the U.S./EU, so patients should verify licensing, cGMP conditions, independent ethics approval, and robust informed consent.
Do I need a visa or insurance for treatment in Colombia?
Many nationalities can enter visa-free for up to 90 days; longer treatments may require a Special Temporary Visa for Medical Treatment (about $50 plus processing time). Standard health insurance rarely covers these therapies—budget out-of-pocket and obtain travel insurance with medical evacuation. Clinics typically provide official documentation to support visa applications and medical necessity.